A closer look:
Millions are affected by DED, but few receive Rx treatment
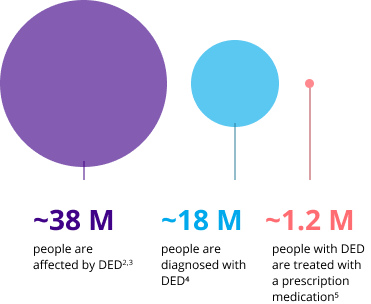
DED represents ~35%-40% of practice across ECP specialties6
ECP, eye care provider; Rx, prescription.
A closer look:
Addressing evaporation is an unmet need in DED7-10
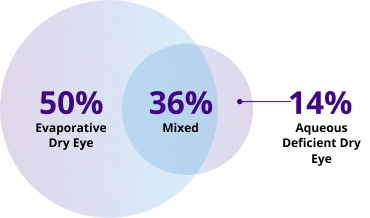
Studies have shown ~86% of patients with DED have excessive tear evaporation associated with meibomian gland dysfunction (MGD)10-12
Dry eye disease is the number–one medical condition that motivates patients to see their ECP—and it will only grow.13
A closer look:
MIEBO is the first and only prescription eye drop for dry eye disease that directly targets tear evaporation1
MIEBO forms a monolayer at the air-liquid interface of the tear film, which can be expected to reduce evaporation1
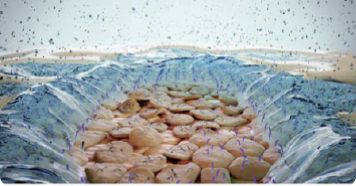
Tear evaporation in DED: Desiccation and damage of the corneal epithelium12,13
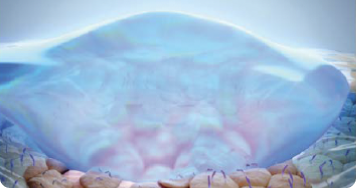
After MIEВО: MIEBO spreads across the surface of the eye12
MIEBO
 Mimics key functions of natural meibum14,15
Mimics key functions of natural meibum14,15
 Promotes healing on the ocular surface1,15
Promotes healing on the ocular surface1,15
 May reduce friction16-18
May reduce friction16-18
Hear directly from leading eye care experts who choose MIEBO for its unique ability to directly target excessive tear evaporation as they discuss its impact in real-world practice.
The exact mechanism of action of MIEBO is not known.
A closer look:
MIEBO delivered consistent results across two large clinical trials1,19
Rapid (as early as Day 15) and sustained (through Day 57) relief of the signs and symptoms of DED1,19
Pooled data from 2 pivotal clinical trials
SIGNS
2x
IMPROVEMENT
vs control in total CFS at Day 571,19
INDIVIDUAL STUDY DATA1,17,18
GOBI: Mean (SD) CFB –2.0 (2.6) for MIEBO (n=289) vs –1.0 (2.7) for control (n=279) (P<0.001) at Day 57.
MOJAVE: Mean (SD) CFB –2.3 (2.8) for MIEBO (n=302) vs –1.1 (2.9) for control (n=296) (P<0.001) at Day 57.
SYMPTOMS
1.5x
IMPROVEMENT
vs control in eye dryness at Day 571,19
INDIVIDUAL STUDY DATA1,17,18
GOBI: Mean (SD) CFB –27.4 (27.9) for MIEBO (n=289) vs –19.7 (26.7) for saline (n=279) (P<0.001) at Day 57.
MOJAVE: Mean (SD) CFB –29.5 (28.6) for MIEBO (n=302) vs –19.0 (27.2) for saline (n=296) (P<0.001) at Day 57.
Pooled analysis: Mean baseline tCFS = 6.9 for MIEBO and saline (control). tCFS grading scale: 0-15 (0-3 in each of 5 areas). Mean baseline eye dryness score = 65.6 for MIEBO, 65.5 for saline (control). Eye dryness Visual Analog Scale (VAS): 0-100 (0 = no discomfort, 100 = maximal discomfort). Across GOBI and MOJAVE, 614 patients received MIEBO and 603 patients received control with 591 and 575, respectively, assessed on Day 57.17-19
Study design: Two 57-day, multicenter, double-masked, saline-controlled studies (GOBI and MOJAVE) were conducted in adults ≥18 years old with a self-reported history of DED in both eyes. Primary outcomes were change from baseline in tCFS and change from baseline in eye dryness score (Visual Analog Scale) at Day 57. Day 15 was the earliest time point at which signs and symptoms were evaluated in the trials. Day 57 was the last.1,17,18
A closer look:
In clinical studies, the majority of patients rated MIEBO as comfortable or very comfortable on instillation19
In 2 pivotal clinical studies with >600 patients treated with MIEBO:
No incidences of serious ocular adverse events (AEs)17,18
Most AEs were considered mild
Low discontinuation rate due to AEs17-19
Discontinuation rate for MIEBO was comparable to control (pooled: 0.2% vs 0.5%; GOBI: 0.3% vs 1.0%; MOJAVE: 0% vs 0%)
Low rate of burning or stinging17-19
The pooled incidence of instillation site pain, such as burning or stinging, was 0.5% (GOBI: 1.0%; MOJAVE: 0%)
One ocular AE with an incidence of ≥2%1,17-19
The most common ocular AE was blurred vision, which was mostly mild and transient. Blurred vision (pooled: 2.1%; GOBI: 3.0%; MOJAVE: 1.3%) and conjunctival redness (pooled: 0.8%; GOBI: 0%; MOJAVE: 1.3%) were reported in 1%-3% of individuals
MIEBO should not be administered while wearing contact lenses. Contact lenses should be removed before use and for at least 30 minutes after administration of MIEBO.
In a 1-year safety extension study, no single ocular AE was reported at an incidence of ≥2%20
Join our insiders’ network, Eyes on Innovation, for exclusive updates. Connect with a B+L account director.
Get updates + Connect with USINDICATION
MIEBO® (perfluorohexyloctane ophthalmic solution) is indicated for the treatment of the signs and symptoms of dry eye disease.
IMPORTANT SAFETY INFORMATION
- MIEBO is contraindicated in patients with known hypersensitivity to perfluorohexyloctane
- MIEBO should not be administered while wearing contact lenses. Contact lenses should be removed before use and for at least 30 minutes after administration of MIEBO
- Instruct patients to instill one drop of MIEBO into each eye four times daily
- The safety and efficacy in pediatric patients below the age of 18 have not been established
- In pivotal trials, the most common ocular adverse reaction was blurred vision (1% to 3% of patients reported blurred vision and conjunctival redness)
Click here for full Prescribing Information for MIEBO.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
References:
1. MIEBO. Prescribing information. Bausch + Lomb, Inc. 2. Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the Beaver Dam Offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157:799-806. 3. US population by age and gender, 2020. 4. 2020 Dry Eye Products Market Report: A Global Analysis for 2019 to 2025. Market Scope. market-scope.com/pages/reports/250/2020-ophthalmic-landscape-report-global-analysis-for-2019-to-2025-april-2021#reports 5. IQVIA data, July 2021. 6. Dry Eye Disease Preference Share Quantitative Research Report. June 2022. 7. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15(4):802-812. 8. Dunn JD, Karpecki PM, Meske ME, Reissman D. Evolving knowledge of the unmet needs in dry eye disease. Am J Manag Care. 2021;27:S23-S32. 9. Kim M, Lee Y, Mehra D, Sabater AL, Galor A. Dry eye: why artificial tears are not always the answer. BMJ Open Ophth. 2021;6:e000697. doi:10.1136/bmjophth-2020-000697 10. Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-478. doi:10.1097/ICO.Ob013e318225415a 11. Badian RA, Utheim TP, Chen X, et al. Meibomian gland dysfunction is highly prevalent among first-time visitors at a Norwegian dry eye specialist clinic. Sci Rep. 2021;11(23412):1-8. doi:10.1038/s41598-021-02738-6 12. Rabensteiner OF, Aminfar H, Boldin I, et al. The prevalence of meibomian gland dysfunction, tear film and ocular surface parameters in an Austrian dry eye clinic population. Acta Ophthalmol. 2018;96:e707-e711. doi:10.1111/aos.13732 13. Karpecki P. The evolution of dry eye. Rev Optomet. January 15, 2015. Accessed November 9, 2022. https://www.reviewofoptometry.com/article/the-evolution-of-dry-eye 14. Sheppard JD, Nichols KK. Dry eye disease associated with meibomian gland dysfunction: focus on tear film characteristics and the therapeutic landscape. Ophthalmol Ther. 2023;12(3):1397-1418. doi:10.1007/s40123-023-00669-1 15. Vittitow J, Kissling R, DeCory H, Borchman D. In vitro inhibition of evaporation with perfluorohexyloctane, an eye drop for dry eye disease. Curr Ther Res Clin Exp. 2023;98:100704. doi:10.1016/j.curtheres.2023.100704 16. Schmid ID, Bata AM, Szegedi S, et al. Influence of perfluorohexyloctane eye drops on tear film thickness in patients with mild to moderate dry eye disease: a randomized controlled clinical trial. J Ocul Pharmacol Ther. 2020;36(3):154-161. doi:10.1089/jop.2019.0092 17. Tauber J, Berdy GJ, Wirta DL, Krösser S, Vittitow JL; GOBI Study Group. NOV03 for dry eye disease associated with meibomian gland dysfunction: results of the randomized phase 3 GOBI study. Ophthalmology. 2023;130(5):516-524. doi:10.1016/j.ophtha.2022.12.021 18. Sheppard JD, Kurata F, Epitropoulos AT, Krösser S, Vittitow JL; MOJAVE Study Group. NOV03 for signs and symptoms of dry eye disease associated with meibomian gland dysfunction: the randomized phase 3 MOJAVE study. Am J Ophthalmol. 2023;252:265-274. 19. Data on file. 20. Protzko EE, Segal BA, Korenfeld MS, Krösser S, Vittitow JL. Long-term safety and efficacy of perfluorohexyloctane ophthalmic solution for the treatment of patients with dry eye disease: The KALAHARI study. Cornea. Published online ahead of print. Accessed June 10, 2024. https://journals.lww.com/corneajrnl/fulltext/9900/long_term_safety_and_efficacy_of.408.aspx
INDICATION
MIEBO® (perfluorohexyloctane ophthalmic solution) is indicated for the treatment of the signs and symptoms of dry eye disease.
IMPORTANT SAFETY INFORMATION
- MIEBO is contraindicated in patients with known hypersensitivity to perfluorohexyloctane
- MIEBO should not be administered while wearing contact lenses. Contact lenses should be removed before use and for at least 30 minutes after administration of MIEBO
- Instruct patients to instill one drop of MIEBO into each eye four times daily
- The safety and efficacy in pediatric patients below the age of 18 have not been established
- In pivotal trials, the most common ocular adverse reaction was blurred vision (1% to 3% of patients reported blurred vision and conjunctival redness)
Click here for full Prescribing Information for MIEBO.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
