
A closer look at a once-daily drop to treat open-angle glaucoma (OAG) or ocular hypertension
Discover a prescription eye drop that delivers powerful and sustained IOP reduction.1-3
IOP, intraocular pressure.
A closer look:
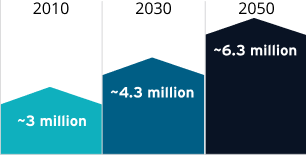
Glaucoma affects ~3 million people in the United States, and that number is expected to rise4
National Eye Institute projections for glaucoma
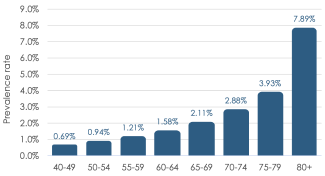
Nearly 80% of all glaucoma in the United States is OAG, with 2 of the biggest risk factors being age and race.4,6,7
Prevalence of OAG by Age Group (2010)4

3x
greater prevalence
of OAG relative to non-Hispanic whites in the United States7
OAG is a chronic optic neuropathy that is usually, but not always, associated with elevated IOP7,8
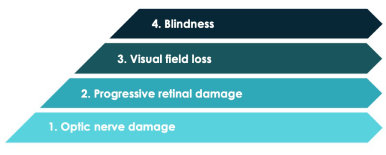
Glaucoma is the second leading cause of blindness in the United States.9
A closer look:
Self-reported visual disability in glaucoma is associated with substantial economic burden10
Glaucoma patients with perceived vision loss have higher medical costs than patients without the perception of vision loss*
Glaucoma with visual disability:
$18,073 (n=1287)
Glaucoma with no visual disability:
$15,829 (n=1952)
Physician services accounted for the highest percentage of costs for both groups
*Longitudinal observational study of 72,587 Medicare beneficiaries conducted in 2004-2009. Costs are US 2015 dollars, adjusted for inflation. (P=0.009)
A closer look:
Lowering intraocular pressure (IOP) has a clinically significant impact on preserving vision7,11-13
Multiple landmark studies have demonstrated that lowering IOP has an impact on disease progression:
Target a 20% to 30% IOP reduction7

The AAO recommends reducing IOP by 20% to 30% below baseline, with adjustments depending on disease course and severity.
Lower progression risk with every 1 mmHg of IOP reduction11

In the Early Manifest Glaucoma Trial, each additional 1 mmHg of IOP reduction in the first 3 months of treatment was associated with an approximate 10% decrease in progression risk.
Reduce IOP to <18 mmHg12,13

In the Advanced Glaucoma Intervention Study, maintenance of IOP <18 mmHg was significantly associated with reduced loss of visual field over long-term follow-up. A goal of <18 mmHg is also recommended for patients with mild to moderate glaucoma.
A closer look:
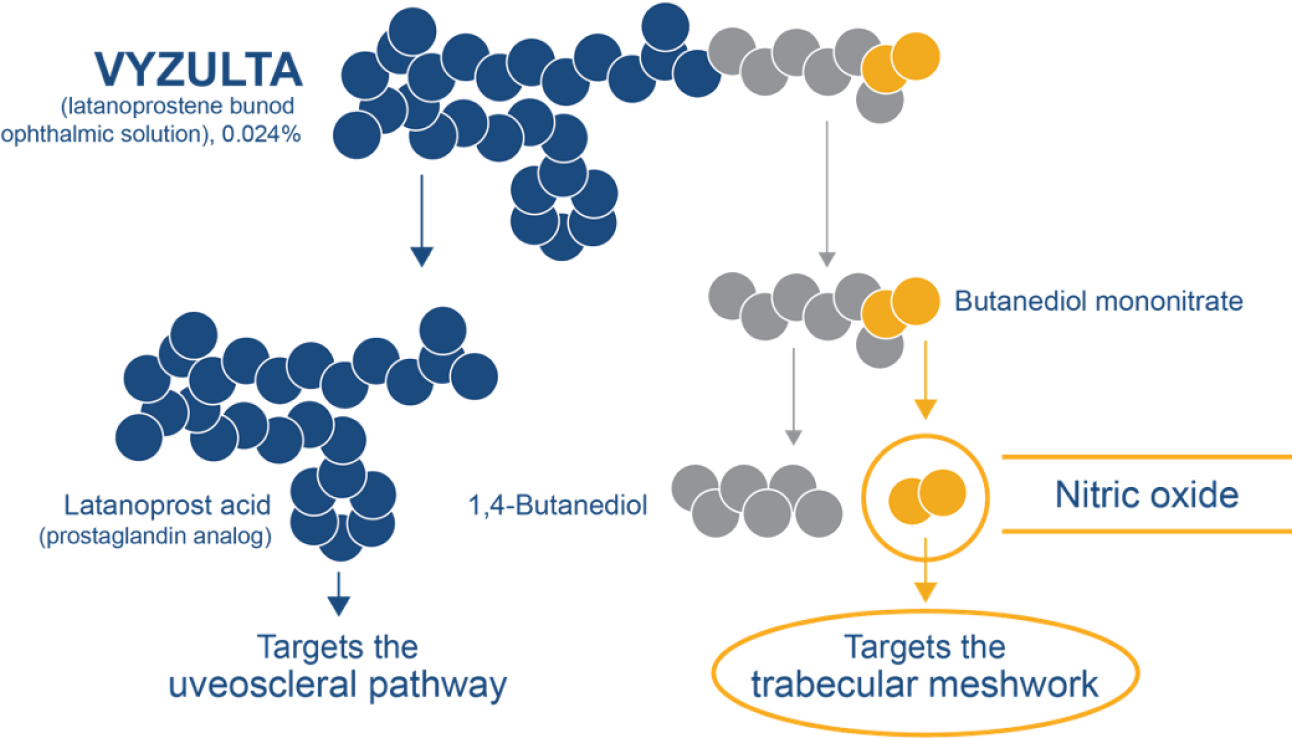
Dual-action VYZULTA—the only nitric oxide-releasing agent for the reduction of IOP14-16
A closer look:
Powerful and sustained IOP reduction1-3
Mean IOP was lower with VYZULTA through Month 3 and stayed low through Month 12 in a pooled analysis17
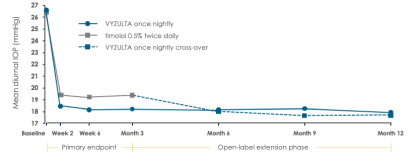
Baseline mean diurnal IOP was 26.7 mmHg and 26.5 mmHg in APOLLO and 26.6 mmHg and 26.4 mmHg in LUNAR for VYZULTA and timolol 0.5%, respectively.2,3
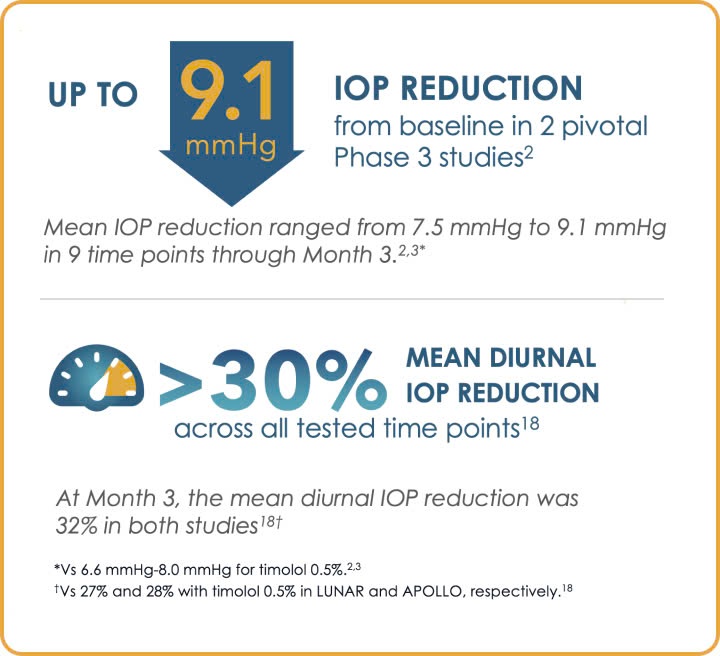
Greater IOP reduction targets1,7,19
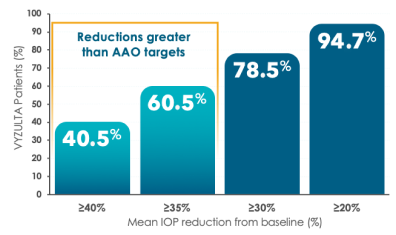
Most VYZULTA patients exceeded AAO IOP reduction targets of 20% to 30%1,7,19
Post hoc analysis: IOP reduction from baseline at Month 31,19
72%
of patients treated with VYZULTA reached ≤18 mmHg at Month 3, meeting the clinically meaningful threshold established in AGIS12,17
Baseline mean diurnal IOP was 26.7 mmHg and 26.5 mmHg in APOLLO and 26.6 mmHg and 26.4 mmHg in LUNAR for VYZULTA and timolol 0.5%, respectively.2,3
Responder rates based on best IOP reduction from baseline out of 3 assessments (8 AM, 12 PM, and 4 PM) at the Month 3 visit. Proportions of timolol 0.5% patients achieving ≥20%, ≥30%, ≥35%, and ≥40% reductions were 91.1%, 62.5%, 44.2%, and 25.3%, respectively.19
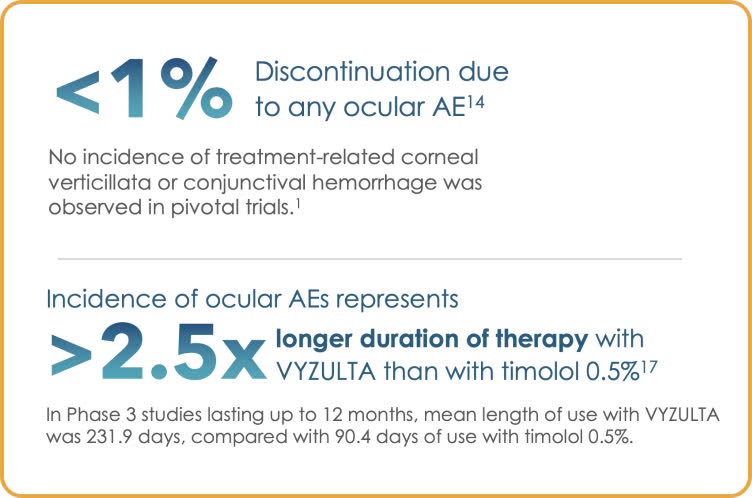
A closer look:
VYZULTA monotherapy offers excellent tolerability with low discontinuation rates
Low incidence of hyperemia and other ocular AEs for patients in Phase 3 trials14,17
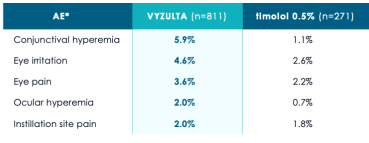
*Pooled results from the APOLLO and LUNAR studies; ocular AEs occurring in ≥2% of VYZULTA study eyes.17
VYZULTA® (latanoprostene bunod ophthalmic solution), 0.024% is indicated for the reduction of intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
Click here for full Prescribing Information.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
References:
1. Data on File. Bausch + Lomb Inc. 2. Weinreb RN, Scassellati Sforzolini B, Vittitow J, Liebmann J. Latanoprostene bunod 0.024% versus timolol maleate 0.5% in subjects with open-angle glaucoma or ocular hypertension: the APOLLO study. Ophthalmology. 2016;123(5):965-973. 3. Medeiros FA, Martin KR, Peace J, Scassellati Sforzolini B, Vittitow JL, Weinreb RN. Comparison of latanoprostene bunod 0.024% and timolol maleate 0.5% in open-angle glaucoma or ocular hypertension: the LUNAR study. Am J Ophthalmol. 2016;168:250-259. 4. National Eye Institute. Glaucoma tables. Accessed June 17, 2024. https://www.nei.nih.gov/learn-about-eye-health/eye-health-data-and-statistics/glaucoma-data-and-statistics/glaucoma-tables 5. Glaucoma. National Eye Institute, National Institutes of Health. Accessed June 17, 2024. https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/glaucoma 6. Sheybani A, Scott R, Samuelson TW, et al. Open-angle glaucoma: burden of illness, current therapies, and the management of nocturnal IOP variation. Ophthalmol Ther. 2020;9:1-14. 7. American Academy of Ophthalmology. Primary open-angle glaucoma Preferred Practice Pattern®. 2020. https://www.aao.org/education/preferred-practice-pattern/primary-open-angle-glaucoma-ppp 8. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901-1911. 9. Gupta P, Zhao D, Guallar E, et al. Prevalence of glaucoma in the United States: The 2005–2008 National Health and Nutrition Examination Survey. Invest Ophthalmol Vis Sci. 2016;57:2577-2585. 10. Prager AJ, Liebmann JM, Cioffi GA, Blumberg DM. Self-reported function, health resource use, and total health care costs among medicare beneficiaries with glaucoma. JAMA Ophthalmol. 2016;134(4):357-365. 11. Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E; Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121(1):48-56. 12. AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429-440. 13. Sihota R, Angmo D, Ramaswamy D, et al. Simplifying “target” intraocular pressure for different stages of primary open-angle glaucoma and primary angle-closure glaucoma. Indian J Ophthalmol. 2018;66(4):495-505. 14. VYZULTA. Prescribing Information. Bausch + Lomb Inc. 15. Buys ES, Potter LR, Pasquale LR, Ksander BR. Regulation of intraocular pressure by soluble and membrane guanylate cyclases and their role in glaucoma. Front Mol Neurosci. 2014;7:38. 16. Cavet ME, Vittitow JL, Impagnatiello F, Ongina E, Bastia E. Nitric oxide (NO): an emerging target for the treatment of glaucoma. Invest Ophthalmol Vis Sci. 2014;55(8):5005-5015. 17. Weinreb RN, Liebmann JM, Martin KR, Kaufman PL, Vittitow JL. Latanoprostene bunod 0.024% in subjects with open-angle glaucoma or ocular hypertension: pooled phase 3 study findings. J Glaucoma. 2018;27(1):7-15. 18. Kaufman PL. Latanoprostene bunod ophthalmic solution 0.024% for IOP lowering in glaucoma and ocular hypertension. Expert Opin Pharmacother. 2017;18(4):433-444. 19. Peace JH, Vittitow JL. Poster presented at: American Academy of Ophthalmology Meeting; October 15-18, 2016; Chicago, IL. Poster PO399.
VYZULTA® (latanoprostene bunod ophthalmic solution), 0.024% is indicated for the reduction of intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
Click here for full Prescribing Information.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.